reorganization energy
from explicit solvation
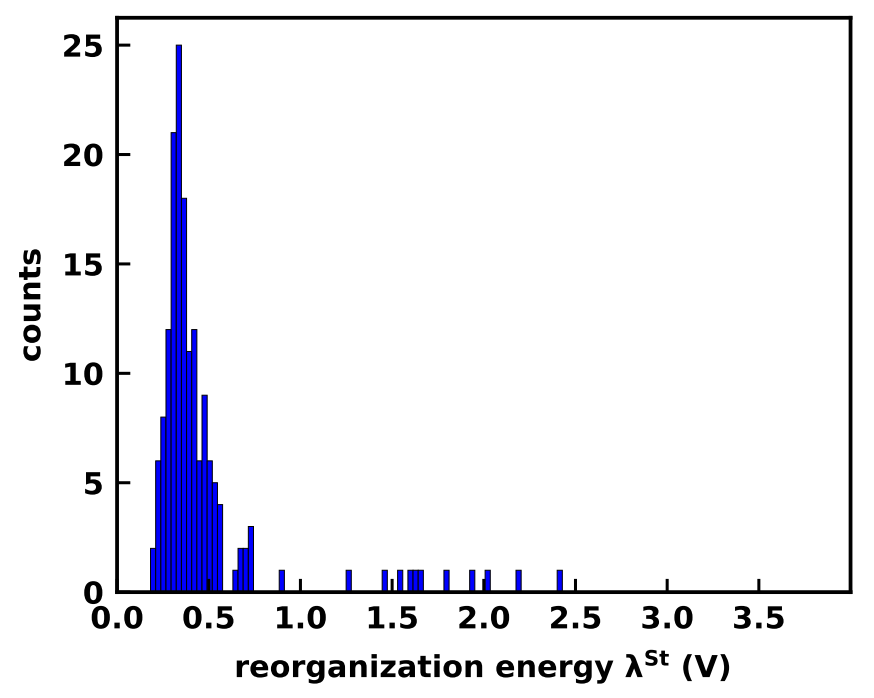
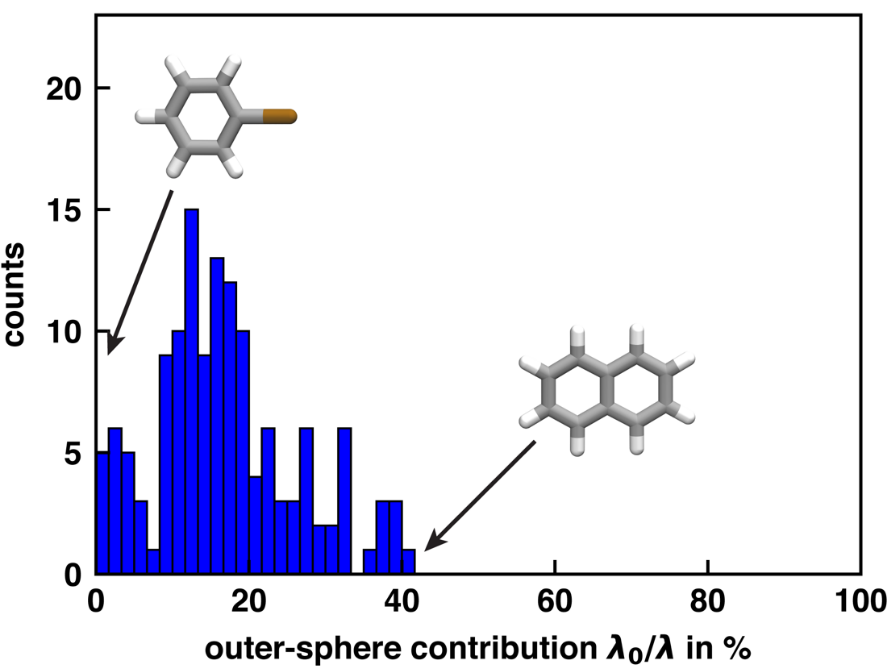
The solvent at room temperature is not static. Chemical changes to the center molecule lead to a reorganization of the solvent, measured as reorganization energy. Previously the computational estimation of reorganization energy was done only for a limited number of systems, but with high-throughput simulation with Autosolvate software described here this could be done for 100+ molecules. The reorganization energy can be split into the contribution by the center molecule and the contribution by the solvent. The fraction caused by solvent (outer-sphere) shown above differs for individual molecules.
[Hruska et al., Bridging the experiment-calculation divide: Machine learning corrections to redox potential calculations in implicit and explicit solvent models, 2022]